Experiment 1
What's the Matter
Introduction to the Physical Properties of Matter
Objective: The objective of this lab is to identify different classes of matter based on physical properties. This lab introduces the key ingredients of concrete. It provides a deeper understanding of the physical properties of concrete.
Scientific Principles:
Matter is divided into the four basic states of solid, liquid, gas, and plasma. Matter is classified based on composition. Homogeneous matter is matter that appears the same throughout a mixture. Heterogeneous matter is matter that has differing appearances throughout the mixture. The concept map below shows the relationship between some of the primary classes of matter.
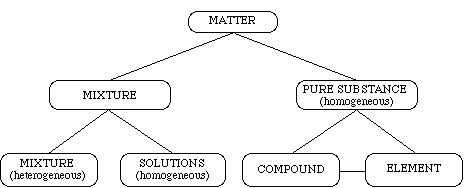
Matter is identified by its characteristic physical properties. Physical properties are those that can be determined without altering the composition of the substance, such as, color, odor, density, strength, elasticity, magnetism, and solubility. Chemical properties are descriptions of the substance and its reactions with other substances to create new substances with new properties. These chemical properties are identified through chemical reactions. Evidence of a chemical reaction possibly occurring can be seen through a color change, temperature change, evolution of a gas, and the formation of a new substance. This lab will only focus on the physical properties of matter.
Time: 45-50 minutes
Materials and Supplies:
- test tubes
- magnet
- magnifying glass
- water
- wooden splints
- Different samples of matter (any of the following):
- baking soda
- balloon filled with air
- iron filings
- flour
- sulfur
- corn starch
- sugar
- vermiculite or perlite
- Styrofoam beads
- salt
- pepper
- cement
- aggregates
- clay
General Safety Guidelines:
Procedure:
- Examine each sample. Record color, odor, and relative particle size in the data table. Use a magnifying glass if necessary.
- With a magnet, test each sample for magnetic properties. Record whether the sample is magnetic or not.
- Test the solubility in water of each sample by adding 5 mL of water to a small test tube. Add some of the sample to the water. Flick the test tube with your finger to help mix the sample in the water. (Note: If mixing does not occur, use a wooden splint.) Record observations.
Data and Calculations:
Fill in the data table based on your observations.
| Sample | Color | Odor | Particle Size | Magnetic | Solubility | State of Matter | Class of Matter |
|---|---|---|---|---|---|---|---|
- a. How do you determine which sample is the most soluble?
b. List the samples from highest to lowest solubility. - Which of the samples would be classified as a mixture?
- What physical properties of matter were tested in this lab?
- What physical properties of concrete would be important to consider when making a structure?
Why?
Notes for Teacher:
- This is a version of a common classification experiment conducted in many chemistry and general science courses. It is included in this module to provide a means for the introduction of concrete and its key ingredients.
- The total number of samples is left to the teacher's discretion. Sand, water, gravel, cement, and concrete should be samples of matter to be tested. This will provide for the introduction of the topic concrete and its key ingredients.
- Be sure the magnet is protected from directly picking up any of the magnetic fragments in the samples. The magnet may be covered with tape or students may place a piece of paper between the magnet and the sample.
- It is strongly recommended that the teacher do a trial run of this experiment before using it with the students.
- This experiment could be used as a lead in to density by asking, "Of the materials that didn't dissolve in water, which was the most dense and least dense?"
- Note that when cement is added to water to determine solubility, the students may conclude that cement is insoluble because its rate of dissolution is relatively slow. However, a quick check of pH will demonstrate that something is happening.
- Note: Vermiculite is a compound.
Answers to Questions:
- a. The sample that has the highest mass dissolved per volume of water is the most soluble.
b. Answers will vary. - This is dependent upon the samples used. However, mixtures are usually obvious from appearance except solutions.
- color, odor, size, magnetism, solubility
- Size of particles and solubility of substances used to make it. The physical properties help determine the purity of concrete's ingredients which greatly affect the produced concrete's characteristics.

No comments:
Post a Comment